|
 
| |
Limathon.com
The
newsletter of
ADS
- LIMATHON Ltd,
UK
June 2006 Edition
Contents
|
NEWS
Ionasys
Collaboration i-BLIPS
FEATURE
History of BLIPS
NEW RELEASES
BLIPS Version 4.0
LIMA Version 6.0
Column Manager 6.0
UPDATES
Latest versions |
Ionasys Collaboration
ADS-Limathon Limited are delighted to announce a
collaborative agreement with Ionasys
Incorporated. In a reciprocal agreement Ionasys will act as sales agents for
ADS-Limathon in the USA, while ADS-Limathon will act as sales agents for Ionasys
in the EEC. Ionasys will also provide offshore software development options to
enable Limathon to bring products to market more quickly and cost effectively.
The first product announced from this collaboration is i-BLIPS.
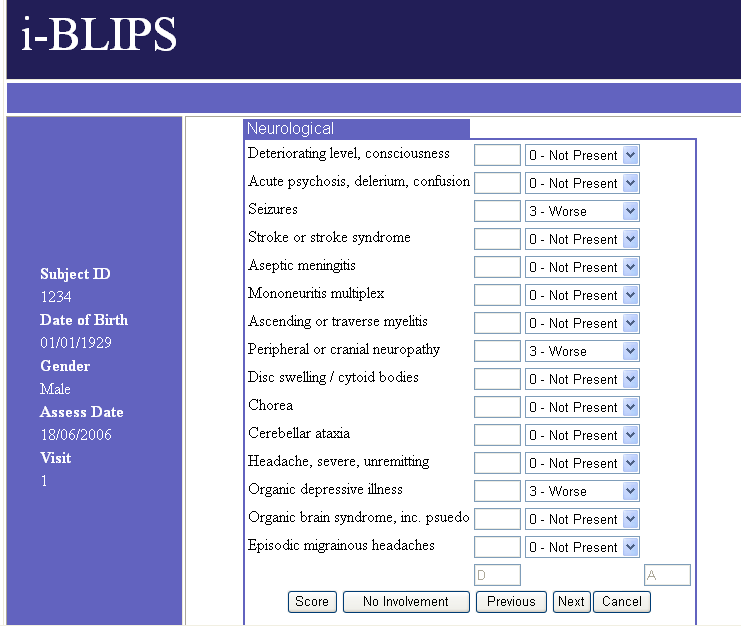
i-BLIPS
In a further agreement, ADS-Limathon have been
working with Ionasys Incorporated to
develop a web enabled version of BLIPS, to be known as
i-BLIPS. Based on
the BLIPS 4.0 calculation algorithm, the product retains many of the familiar
features of BLIPS, but instead of being MS Access based, it is written in Java,
and runs TOMCAT and a MYSQL database, located on a hosted server. This will be
initially aimed at Clinical Studies but it may also prove valuable to
collaborating clinics such as BILAG and SLICC. It provides the Study Organisers
all the major benefits associated with internet applications, including a
central database and a simple front end deployment in Internet Explorer. No
installation on the users PC is required. After the user has been registered in
the database, it is a simple task of entering the web address, and logging on
with a user name and password.
The development is progressing well and a 'Proof
of Concept' product is already available for demonstration. The next steps
involve testing the calculation algorithms in order to validate the software
before it can be deployed to a secure server for use in a live study.
History of BLIPS
The
British Lupus Integrated Prospective System, or BLIPS as it is known, is the
result of 10 years of collaboration between British Isles Lupus Assessment Group
(BILAG) and ADS.
Since 1984, the BILAG group has been monitoring thousands of
patients with the autoimmune disease Systemic Lupus Erythematosus (SLE). Many
authoritative clinical papers have emanated from the group and they have
documented a complex index for assessing and controlling the disease. For ADS,
the project started out in 1996 as a simple data migration of a legacy BILAG
database into MS Access 2.0. Such is the power of Access, it ended up four years
later as a highly sought after tool for Lupus researchers worldwide.
BLIPS Version One was released
exclusively to the Bilag group in 1998 as a direct replacement for their old DOS
BILAG database. It included a completely new GUI interface for the Bilag index
– monitoring of eight clinical systems – mucocutaneous, immunological,
vascular, haematological, cardiovascular, biochemical neurological,
musculoskeletal, and added new functionality recording of medication drugs and
laboratory results.
BLIPS Version Two in 1999 added two further
internationally recognised indices – SLICC and SF-36. Graphing of results was
introduced to the functionality, and the product became a network client-server
application.
Blips Version 3.0 was released worldwide in 2000
allowing non-BILAG clinics to obtain the tool. Version 3.0 included two more indices, SLAM-R and Selena Sledai. It was recoded in Access 97
and a 100-page user guide and helptext were added. BLIPS 3.0 is now in use in
major universities and hospitals throughout the UK, USA and Canada and as far a
field as China, Korea, Australia and the Philippines. During
2003-2005 several clinical studies were conducted using BLIPS 3.0
BLIPS Version 4.0 was released first released in a
bespoke Clinical Study version in November 2005.
It has since been used introduced in more studies in
2006. Changes included improved security and
audit trail for FDA CFR21 compliance. Demographic data screens were replaced
with Patient IDs. Sign off reports were introduced, along with the ability to
automatically email them to the Study Control centre. A system admin function
was introduced to allow Study Organisers to define which functionality would be
available to clinics during trials. Further development has enabled BLIPS
4.0 to be released in June 2006 as a standard product release, rather than
restricting it to Clinical Studies.
BLIPS
is now in use in over 30 countries around the world, and since release around
200 users have registered their copies.
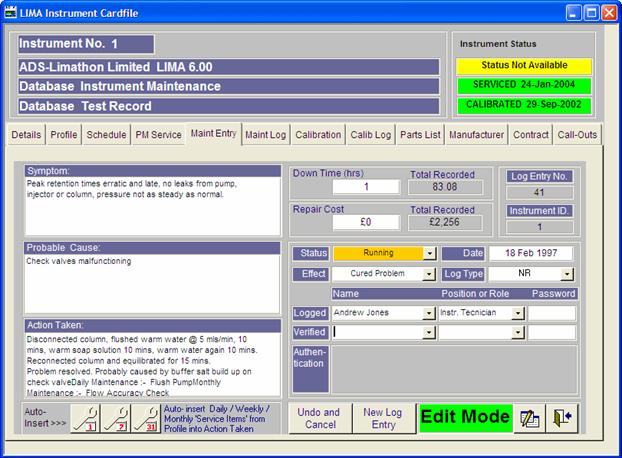
LIMA™ Version
6.0
The industrial diversity of the LIMA customer base
continues to motivate us here at ADS-Limathon.
LIMA Version 4.0 and its Network Administrator Version
was the result of collaborative work with food and confectionary giant,
Nestle, in Switzerland.
LIMA Version 5.0 , the Service Base version, was a
developed in collaboration with DP Services a petrochemical instrument
maintenance company in Scotland. This version was specifically designed for use
by Instrument Maintenance companies and includes a customer database and is
still available.
And now LIMA 6 has been developed in collaboration with
the National Thermal Power Corporation in India.
The new version has increased FDA 21CFR compliance
with electronic signature validation and stricter logon, and
complete audit trail. Enhancements specified by NTPC included improved
calibration settings allowing automatic generation of calibration points between
the upper and lower limits, based on either % of upper value, or % of Range. The
concept of Master Calibration Instruments is also catered for, along with
improved sign off reports. Other improvements designed by ourselves were focused
on enhancing the Admin sections introduced in version 4 and extending the use of
tab screens to the instrument record card.
ADS-Limathon Managing Director, Gordon Hamilton,
commented : "LIMA had grown from a simple and easy to use instrument log
database into a very complex and flexible instrument management solution. The
challenge was to increase the complexity yet again, retain the flexibility to
support divers industries, but streamline the user interface to improve ease of
use and user navigation. Early comments from customers suggest we have achieved
this. LIMA has been around now for 10 years so its a fantastic accolade to
hear its still a top solution. The introduction of features required by the
power industry is already paying dividends as the International Atomic Energy
Agency based in Vienna has committed to purchase LIMA for over 20 atomic power
stations in Africa. Equally satisfying is the knowledge that while LIMA was
originally designed for the Laboratory industry ten years ago, it has been
reshaped to support food, petrochem and electricity industries, but it has
retained its support in its native sector. Last week a new customer at A.P.
Pharma, (USA) told to us last week that after reviewing all the products on the
market, LIMA was by far the best solution for her Pharmaceutical Laboratory."
International Atomic Energy Agency is the latest in a
long list of valued internationally famous customers using LIMA, which includes
the US Navy (at Pearl Harbour), Esso, Exxon, Heineken, Pfizer, Procter & Gamble,
Nestle, General Electric (to name a few !).
For the technophiles LIMA Version 6
is available with Access XP Runtime as a stand alone PC version. Network
versions will require Access XP. Access 2000 and 2003 versions are also
available upon request. The upgrade route from LIMA 2000 or LIMA 4 involves data
migration which ADS-Limathon can provide upon request.
The above example report shows the
results of a generic search for ADS-Limathon’s LIMA product using the keywords
“laboratory instrument calibration database. Forty search engines now place
ADS-Limathon at number one for this search, and a further four in the top ten.
None of these listings are paid inclusions.
If you need your web site designed,
redesigned, hosted, submitted, analysed or optimised then contact ADS-Limathon
now.
HPLC Column Manager™ Version 6.0
A major column manufacturer once
described this software as “the ultimate computerised tracking system for HPLC
columns”. ADS-Limathon are pleased to announce the new version 6.0 of HPLC
Column Manager™. This Windows™ database solution comprises an inventory
specifically for HPLC columns and provides relational storage for recording
audit trail on all your HPLC columns, as well as informational items such as
suggested use, restrictions on use, user identification, dated performance
history, and validation test results and chromatograms. New features in Column
Manager Version 6.0 are the abilities to store multi-component column tests and
record multi-component column validations. New graphing options allow graphing
of any performance parameter by test and by analyte.
Related HPLC reference databases are included, providing data
at a click, on over 1800 columns, 4000 applications (optional) and HPLC
troubleshooting databases, HPLC Column Manager™ can be used with all brands of
HPLC columns and is compatible with all Windows™ based chromatography data
systems. The new Version 6.0 of HPLC Column Manager™ will start shipping in
April 2002. Special upgrade packages will be available to existing users of
Column Manager 3.0, 4.0 and 5.0 shortly afterwards.
A special vote of thanks to Column
Manager users Tony Polletta at Boeringer (USA) and Trevor Chapman at Pfizer (UK)
who helped to identify improvements necessary for CM v6.0.
Still
Using Column Manager v3.0 or v4.0 ?
Version 5.0 gave chromatographers an
improved user interface with much faster and more flexible navigation. Besides
the standard version 3.0 functionality of column inventory, and column audit
trail, new features have been added. All screens now have custom menus. You can
copy the details of an existing column for a new record. A built in calculator
will convert your column measurements into theoretical HPLC factors, a reference
chromatogram can now be stored and improved graphing.
Current
Software
Versions/Revisions
- BLIPS v4.0 Revision
4.05
-
LIMA v6.0
Revision
6.08
- RAID v2.0 Revision
2.15
- Column Manager
v5.0 Revision 5.12
- Column Manager v6.0 Revision
6.04
- SOPmanage v3.0
Revision 3.4.0
- New templates
available for Word 97 2000, XP, 2003
- Reference
Manager v2.0 Revision 2.0
Upgrades to the latest software
revision are available free of charge to users who have current LimaCover
Contracts. For users who do not have current contracts but would like the latest
software revision, there is a charge.
Major software version upgrades are
available at a discounted price to users with current LimaCover contracts.
Flexible Friendly Products !
ADS - Limathon are now pleased to offer the
facility to purchase products and services using most major credit cards.
However we urge customers to fax their card
details to us. In the interest of your own security do not email you card
details to us

Orders are still accepted by providing a
Purchase Order, Cheque with order, or by e-mail. Delivery is generally within
7-10 days unless customisation is required. Payment terms are 28 days from
invoice date.
ADS-Limathon
Ltd
3 Ringwood
Road
Sheffield,
S20 2DG, UK
Tel:
+44 (0) 114 251 0820
Fax:
+44 (0) 114 247 5771
Web:
www.limathon.com
|
