       
Chromview - A Commom Platform for Storage, Retrieval and Exchange
of HPLC
Methodology and Chromatograms
Technical Article
10 February 2000
It is incredible to think that
the first aircraft was developed as recently as 1903, and today modern airliners
are designed, built and virtually controlled by computers. In that same year the
first chromatography was developed. Yet today as we approach the 21st century,
if we are honest with ourselves, we as chromatographers are still thumbing
through column manufacturers catalogues for documented methods, then we modify
or refine them by hit and miss techniques.
Despite chromatography data
integration having almost completed its migration from the chart recorder and
integrator, to the PC Data Station and LIMS environment, one glaring omission of
even the latest data systems is the inability to archive chromatography
methodology in a standard database format which can be exchanged, stored, viewed
and searched by anybody regardless of who manufactured the integration package
or system hardware. Computers were handling chromatography data long before the
PC became common in many offices or homes. So why is it I can send a document,
spreadsheet or multimedia presentation to another PC user to view, but I cant
send a chromatogram or application note electronically.
This situation exists
probably for any permutation of three reasons.
Firstly computerisation in
chromatography has - quite rightly - focused on improving laboratory efficiency
through obtaining better analytical accuracy and higher throughput of samples
while consuming less of the analysts time.
The second reason is perhaps
that major chromatography manufacturers appear to be. playing a game of
technological leapfrog with no set rules or boundaries. Can you imagine the
chaos if Heathrow airport changed its radar system and software every year and
all airlines played catch-up ?
The third reason is that
software development and support is expensive and not highly profitable to
instrument manufacturers. Some would secretly admit that they would be happy to
pull out of the software market if it was not for the fact that the software is
needed to sell their instruments !
In short, developing
non-instrument-related software is not high on instrument manufacturers agendas!
So where does this leave the
method developer who wants to search for a method a colleague developed two
years ago? Where does this leave the analyst who needs to start developing a new
method from scratch ? Where does leave a methods development department who want
to share their work with QC department or with a different site of the same
company. Integration software can not address this issue. Even if both sites
have the same manufacturers data handling they may be able to read each others
methodology but it is still impractical to search through endless data files to
find a method.
HPLC column manufacturers,
in general, have always been generous with publishing and sharing their tried
and tested methods with the world at large. Call me Mr Cynical, but this may be
something to do with the fact that it is sells their columns. Even as the column
manufacturers start to move their hard copy methodology archives towards an
electronic format, the probability is high that most of them will develop their
own totally incompatible databases, on CD-ROM. The end result will be that the
poor old chromatographer has to browse through stacks of CD-ROM’s instead of
catalogues. Many people already believe its quicker to thumb a catalogue than
fumble with a CD-ROM , then wait till it loads the program and data. What’s
more you can probably guarantee that these programs will not allow you to add
your own developed methods to the database or import methods from any other
manufacturers database. It’s not a great solution for the chromatographer !
So what does the
chromatographer really, really want ?
Storing Application
Methodology
The database must be capable
of storing all aspects of the chromatographic methodology, Analytes, Matrix,
Column, Solvents, Gradient, Detection, Validation Notes, references etc. A
relational database is required for storing these details efficiently and in a
relational manner.
Depicting Chromatograms
The chromatogram must be
stored in the database as a vectored image, either an OLE object, or a metafile
using Windows Clipboard cut-and-paste. Almost all Windows data-stations support
copy to clipboard and many data stations allow export of single data files as
AIA (Andi) files. AIA files can now be converted to OLE objects which are
extremely efficient in file size (eg 30K). One of the major obstacles to storing
the chromatogram in a PC database is the picture file size. This must be
minimised. Scanned chromatograms are not a good option due to large file size (eg
300-500K) and the inability to resize the non-vectored image. Large picture file
size are the most common reason for current databases appearing on CD-ROM.
Searching
It is the purpose of all
databases to store data. However it is only a good database which retrieve the
required data easily and present it in a manner which is useful to the user.
Chromatographers have
differing requirements to search for methods. Some may prefer to browse all
applications containing a named analyte while others may wish to find an
application containing one analyte in the presence of other named analytes.
Pharmaceutical method developers often do not even have the luxury of a named
compound, and chemical structure, sub-structure or smile string may be the
preferred method of locating relevant applications.
Data Entry
To be a useful tool to any
method developer the database must permit entry of the chromatographers own
methods. This is where the CD-ROM approach used by some manufacturers falls
down. Such databases are closed systems and as such are often consigned to the
shelf where the catalog used to reside.
Import of Data
If the chromatographer has
the ability to write methods to the database, he must be able to import new
methods from other departments or sites within his company, and other sources
outwith his company, for example column manufacturers. It is not sufficient to
replace an old database with a new version since any data the chromatographer
has added will be overwritten.
Hard Disk Storage
The chromatographer needs a
single tool which is stored on his hard disk and is capable of storing his own
developed methods alongside other (imported) methods. The data may reside on a
local PC or a network server, and should be readily available to use. Scouring
multiple CD-ROMs should not be an acceptable solution with today’s technology.
Data Sharing
Unbelievable as it may
sound, I personally know of one large pharmaceutical company where analysts in
one lab were trying to develop a method which had been developed the previous
week by colleagues in a lab at the other end of the corridor. Furthermore, how
many analysts know there is a method somewhere, but can not find it amongst the
mountains of accumulated hard copy and gigabytes of raw data ! Or maybe the
method was developed by somebody who left the company, who was it, where is the
method stored ? We’ve all witnessed at least one of these scenarios, I’m
sure! The solution to these types of problems is a shared database. Current
database technology allows data tables and database engines to be installed
separated. By storing the database tables on a central server and installing the
engine at PC terminals, all chromatographers can access, add, search and
retrieve the data. By using database security or modifying the database engine
certain users can have limited access - for example read only access- while
others can read and add data.
Operating Platform
The overwhelming majority of
chromatography operating systems are PC based. The operating platform should
therefor be, ideally, Microsoft based, or Microsoft compliant and or compatible.
It should operate on Windows, Windows 95 and Windows NT platforms. There should
e a common upgrade route betwwen these platforms. It should be capable of
running on a stand alone PC and also it must also be able to run over common
network protocols in both local area and wide area network. Again an upgrade
from single user to network must be available.
To Summarise - the ideal
solution is a database
- · which can store
application methodology
- · which can store and
display chromatogram object
- · which accepts
chromatograms from any PC datastation
- · that can search and
retrieve by component names
- · that can store and
preferably search chemical structures
- · that permits the addition
of users own methods
- · that permits the
importing of more data
- · that lives on a hard disk
or server, not a CD-ROM
- · that allows export of
methods to be imported/viewed by others
- · which can be networked
using LAN or WAN
- · Is Microsoft compliant
and operate on Windows, Windows 95 or Windows NT
The Solution
A solution to meet the above
chromatographers’ specifications clearly can not be produced overnight! From
experience we know it takes about three years to develop and refine such a
database system. The good news is we started in 1994 and we now have a solution
to meet the above is proposed below.
This solution is written in
Microsoft Access. This means that it is 100% Microsoft Windows compliant and is
based on a database engine written by the worlds largest and most respected
software company. Microsoft have documented upgrade routes from 16-bit engines
to 32-bit database engines and indeed both may be accessing the same database
tables simultaneously. It is also proven as a network solution and can be easily
upgraded from PC to network versions. Access Run-Time is available from licenced
Microsoft Solution Developers which means that the user does not need to have a
licence for Access.
The program is available as
in four versions
1. Chromatography
Application Methods database - PC Version - named FastCAM 2™;
2. Network Version - named
FastCAM™
Network
3. Chromatogram Viewer Only
- Named ChromView™
4. Data Library of Columns
and Application Profiles - Named ReCAP™
· An Optional Program to
convert AIA files to OLE is also available -ChromMerge™;*
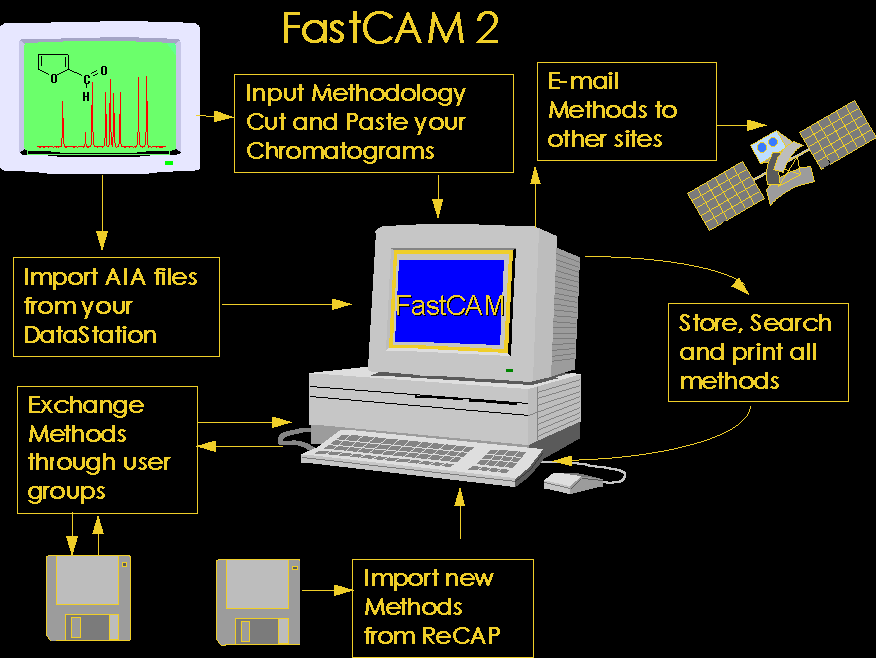
1. FastCAM 2
FastCAM provides facility to
enter methodology, store chromatograms, store chemical structures search, review
and print all methodology. It also permits import of other FastCAM databases and
export of methods to other users who may import them.
Search facilities include :
Application title search, Component name search, multiple component name search,
ChromSearch and ChemSearch (locate on chemical functional groups)
2.
FastCAM Network
This provides all the
facilities of FastCAM for network users. All users connect to the same data
tables allowing access to shared data .
3.
ChromViewer
This provides a low cost
option for users who need to view methods but are not interested in adding their
own data. This can be used on a PC or network. Import facility allows more
applications to be added but there is no write or export facility. Possible uses
are to view applications released by column manufacturers in this format , or as
a read only teminal on a network.
4. ReCAP
This provides 5500
applications with chromatograms and methodology, an index of 20000 components
plus an integrated database of 1800 column materials. Data independantly
compiled from applications sourced from column manufacturers, ensuring proven
methods ta on the latest column technologies.
Both these databases are
available built into ChromViewer or FastCAM. Planned updates will import into
the original programs.
Where can users obtain
applications for their databases ?
There are many potential
sources to obtain data for your methodology database.
- 1. Use FastCAM to enter your
own data
- 2. Export/Import facilities
allow you to exchange your data :
- with
other departments sites of your company,
- with
users groups
- through
e-mail
- 3. Limathon hope to sponsor
a users ‘swap-shop’ on the internet shortly
- 4. Limathon have 5500
applications already on file which can be imported
- 5. Limathon can provide a
confidential service to produce vector image databases from your archived hard
copy methodology
- 6. Several column
manufacturers in USA, Europe, and Japan have already expressed interest or have
already committed to having databases produced in ChromView format for royalty
free distribution.
TRADEMARKS
Windows and Access are
registered trademarks of Microsoft Corporation
ChromMerge is a registered
trademark of LC Resources Inc
FastCAM 2, ChromView, ReCAP
are trademarks of Limathon Limited
Background Information
Gordon Hamilton worked in
pharmaceutical / chemical analytical and method development labs (Glaxo, BACo,
Johnson Matthey) for ten years before working with LDC, Ciba Corning (Jasco),
Spectra Physics, and Capital HPLC in Sales and Marketing roles in HPLC. He also
spent a year with a chemical reaction database company where he learned database
development before forming his own company in 1994. Limathon Limited produces
chromatography databases for HPLC Column Management, HPLC Reference, HPLC
Application Storage, Instrument Maintenance and Calibration Scheduling, which
are in use in most major pharmaceutical companies in the USA and Europe.
For More Information Contact:
ADS-Limathon Limited
3 Ringwood Road, Sheffield, S20 2DG, UK
Tel: +44 (0) 114 251 0820
FAX: +44 (0) 114 247 5771
Internet:
[email protected]
| 